Early medical response (ECR) is a brand new endpoint to decide whether or not a drug needs to be authorized for community-acquired bacterial pneumonia in the United States.
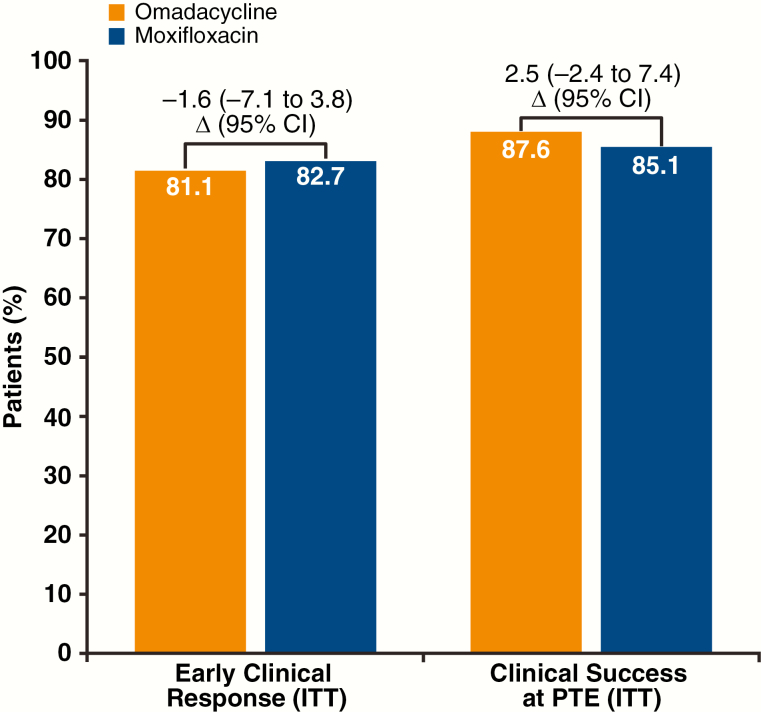
The Omadacycline for Pneumonia Treatment In the Community (OPTIC) section III research demonstrated noninferiority of omadacycline to moxifloxacin utilizing this endpoint. This research describes the efficiency of the ECR endpoint and medical stability relative to a posttreatment analysis (PTE) of medical success.ECR was outlined as symptom enchancment 72-120 hours after the primary dose of research drug (ECR window), no use of rescue antibiotics, and affected person survival.
Clinical success at PTE was an investigator evaluation of success. Clinical stability was outlined based mostly on important signal stabilization, described in the American Thoracic Society and Infectious Diseases Society of America community-acquired pneumonia therapy tips.
During the ECR window, ECR was achieved in 81.1% and 82.7% of omadacycline and moxifloxacin sufferers, respectively. Similar numbers of sufferers achieved medical stability in every therapy group (omadacycline 74.6%, moxifloxacin 77.6%). The proportion of sufferers with improved signs who have been thought-about medically secure elevated throughout the ECR window (69.2-77.6% for omadacycline; 68.0-79.7% for moxifloxacin).
There was excessive concordance (>70%) and excessive optimistic predictive worth (>90%) of ECR and medical stability with general medical success at PTE.Omadacycline was noninferior to moxifloxacin, based mostly on a brand new ECR endpoint. Clinical stability was equally excessive when measured in the identical timeframe as ECR.
Patients with Idiopathic Membranous Nephropathy: A Real-World Clinical and Economic Analysis of U.S. Claims Data.
Membranous nephropathy (MN) is a typical explanation for nephrotic syndrome in nondiabetic adults. Approximately one third of sufferers with MN progress to end-stage renal illness (ESRD), whereas others could also be efficiently handled to remission. Patients with MN symbolize a high-risk inhabitants for whom administration methods can alter and enhance outcomes. Currently, there’s little real-world proof relating to the burden of MN on well being plans.
To (a) characterize medical and financial outcomes throughout a 1-year timeframe amongst a prevalent cohort of sufferers with MN and (b) evaluate the 5% of sufferers incurring the best price with the remaining 95%.A retrospective evaluation of commercially insured sufferers was performed utilizing MarketScan administrative well being care claims knowledge from January 1, 2012, to December 31, 2015. Patients have been aged ≥ 18 years, enrolled In a fee-for-service plan, and had ≥ 2 medical claims for an MN prognosis (ICD-9-CM codes 581.1, 582.1, and 583.1).
Diagnoses indicating clear secondary causes have been excluded wherever doable. Demographics have been decided as of the primary prognosis date; medical traits (e.g., MN-specific remedy, problems, and procedures), well being care useful resource utilization (HCRU; inpatient, outpatient together with different outpatient and emergency division [ED], and prescriptions), and prices have been evaluated for 1 yr following MN prognosis.
Total prices and price distribution (2017 U.S. {dollars}) have been examined utilizing plan-paid and patient-paid quantities. The 95th percentile was used to categorize and evaluate the subcohorts: high-cost cohort (HCC) sufferers (prime 5%) and non-high-cost cohort (NHCC) sufferers (the remaining 95%).
Descriptive analyses, chi-square exams, and Wilcoxon rank-sum exams have been performed.2,689 sufferers have been recognized (60.0% male, imply age = 46.Four years). Severity and superior illness have been noticed In the next proportion of HCC sufferers (n = 134) versus NHC sufferers (n = 2,555) through hostile well being outcomes, procedures, and immunosuppressant use. HCC sufferers used considerably extra assets on common than NHCC sufferers (further use): 1.7 inpatient, 1.2 ED, and 4.
Eight outpatient workplace visits; 15 prescriptions; and 64.Eight different outpatient visits (i.e., outpatient, hospital, and ESRD services). Total MN-related price and imply (SD) price per affected person have been $123.2 million and $45,814 ($101,353); HCC sufferers accounted for 43.7% of whole prices for a imply price per affected person of $401,608 versus NHCC sufferers at 56.3% and imply price per affected person of $27,154. The biggest prices for each teams have been associated to outpatient visits (HCC = 46.7%; NHCC = 52.8%), inpatient visits (HCC = 27.7%; NHCC = 28.6%), and prescriptions (HCC = 25.7%; NHCC = 18.6%).
Patients with MN are considerably burdened with excessive illness severity and hostile well being outcomes, ensuing In substantial HCRU and prices. Health plan price drivers for MN (HCC and NHCC sufferers) occurred primarily In the outpatient setting, adopted by the inpatient setting and prescriptions.
Modifiable points previous development to superior renal illness and worse outcomes needs to be explored to Identify efficient interventions and enhance useful resource allocation earlier In the illness pathway, earlier than ESRD.
This research was funded by Mallinckrodt Pharmaceuticals. Kirkemo, Pavlova-Wolf, and Bartels-Peculis are staff and stockholders of Mallinckrodt Pharmaceuticals.
Nazareth was an worker of Mallinckrodt Pharmaceuticals on the time of this research. Kariburyo, Xie, and Vaidya are staff of STATinMED Research, a paid guide to Mallinckrodt Pharmaceuticals. Sim acquired an investigator-initiated analysis grant from Mallinkcrodt Pharmaceuticals. A portion of the research outcomes have been beforehand introduced on the AmericanSociety of Nephrology (ASN) Kidney Week 2017; November 2, 2017; New Orleans, LA.
