The correct measurement of blood stress (BP) in being pregnant is important to information medical determination making that impacts each mom and fetus. The intention of this systematic assessment was to find out the accuracy of ambulatory, dwelling, and clinic BP measurement gadgets in pregnant girls.
We searched Ovid MEDLINE, The Cochrane Library, EMBASE, CINAHL EBSCO, ClinicalTrials.gov, International Clinical Trials Registry Platform, and dabl from inception by means of August 3, 2017 for articles that assessed the validity of an higher arm BP measurement system in opposition to a mercury sphygmomanometer in pregnant girls.
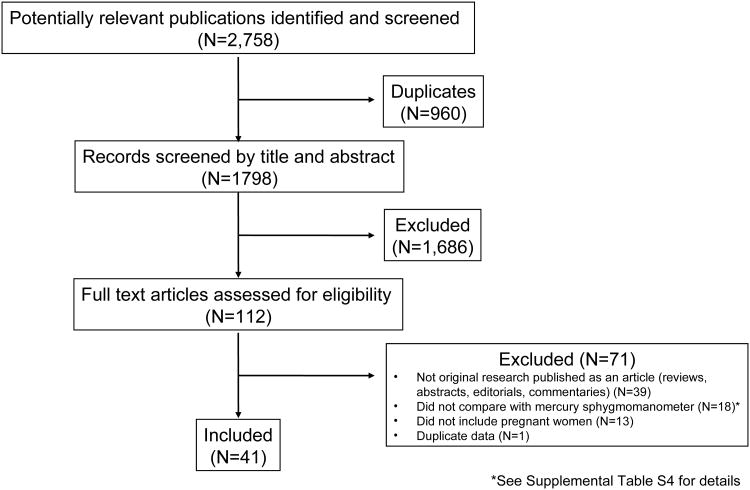
Two impartial investigators decided eligibility, extracted knowledge, and adjudicated protocol violations. From 1798 potential articles recognized, 41, that assessed 28 gadgets, met the inclusion standards. Most articles (n=32) adopted a normal or modified American National Standards Institute/Association for the Advancement of Medical Instrumentation/International Organization for Standardization, British Hypertension Society, or European Society of Hypertension validation protocol.
Several articles described the outcomes of validation research carried out on>>1 system (n=7) or in>>1 inhabitants of pregnant girls (n=12), comprising 64 pairwise validity assessments. The system was validated in 61% (32 of 52) of research which used a normal or modified protocol.
Only 34% (11 of 32) of the research whereby the system was efficiently validated had been carried out and not using a protocol violation. Given the implications of inaccurate BP measurement in pregnant girls, healthcare suppliers needs to be conscious of and attempt to use the BP measurement gadgets which have been correctly validated in this inhabitants.
Mechanical recanalization in basilar artery occlusion: the ENDOSTROKE research.
OBJECTIVEA research was undertaken to judge scientific and procedural components related to consequence and recanalization in endovascular stroke remedy (EVT) of basilar artery (BA) occlusion.
METHODSENDOSTROKE is an investigator-initiated multicenter registry for sufferers present process EVT. This evaluation contains 148 consecutive sufferers with BA occlusion, with 59% having obtained intravenous thrombolysis previous to EVT.
Recanalization (outlined as Thrombolysis in Cerebral Infarction [TICI] rating 2b-3) and collateral standing (utilizing the American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology collateral grading system) had been assessed by a blinded core laboratory.
Good (reasonable) consequence was outlined as a modified Rankin Scale rating of Zero to 2 (0-3) assessed after a minimum of Three months (median time to follow-up = 120 days).RESULTSThirty-four p.c had good and 42% had reasonable scientific consequence; mortality was 35%.
TICI 2b-Three recanalization was achieved by 79%. Age, hypertension, National Institutes of Health Stroke Scale scores, collateral standing, and the use of magnetic resonance imaging previous to EVT predicted scientific consequence, the latter Three remaining impartial predictors in multivariate evaluation. Independent predictors of recanalization had been higher collateral standing and the use of a stent retriever.
However, recanalization didn’t considerably predict scientific consequence.CONCLUSIONSBeside preliminary stroke severity, the collateral standing predicts scientific consequence and recanalization in BA occlusion. Our knowledge recommend that the use of a stent retriever is related to excessive recanalization charges, however recanalization by itself doesn’t predict consequence.
The function of different modifiable components, together with the selection of pretreatment imaging modality and time points, warrants additional investigation.